Under Pressure: Pharma in the Storm

Galenisys Newsletter : June 2025
Table of Contents
By Tony Dunford
Editor
-
The President vs. Big Pharma (as of mid-May!)
By the Editor -
FACING DISASTER: Knowledge and Experience Count
By Bill Torpey -
Due Diligence versus Audit/Inspection
By Richard van Duyse -
Flying blind? Less tracking and forecasting.
The Editor
The President vs. Big Pharma (as of mid-May!)
By the Editor
On the 12th of May, President Trump signed an executive order, aimed at drug makers, to reduce their prices in America to the lowest rate they charge in other rich countries.
That's a second headache for the industry given Mr Trump's trade protectionism. (Although drugs were excluded from the sweeping reciprocal tariffs he announced on the 2nd of April, the duties remained on the table).
As expected, and also because it's true, the industry pointed out that the combined effect will screw up supply chains, raise treatment costs, and slow the development of new medicines.
The executive order instructs his team to:
1. Tell companies the “target prices” that he expects based on international benchmarks.
2. Establish a mechanism for patients to buy drugs directly, not via middlemen. (Cue dodgy internet trade!).
3. And warns of “aggressive measures” - although what these are is unclear.
The problems with the plan are as follows.
Firstly, generic drugs, which make up 90% of prescription volume in America cost around a third less than any other rich country. (To be fair, branded drugs in the USA are more than four times as pricey as in comparable markets). But putting prices down in USA is like sitting on one end of a balloon - the other end - the prices in other parts of the world, will go up, as the industry has to find ways of recouping the costs of its long-term investments in R&D.
Then there are actual hurdles for the orders to take effect:
1. One of these is that the courts may strike down the proposal.
2. Drugmakers can be expected to challenge the effort.
3. Implementation issues are serious as well, partly because of the length & complexity of supply chains. These are global & hence tariff vulnerable, having been designed not only to reduce manufacturing cost, but taxes as well. Invoices as well as the actual materials can travel through a variety of jurisdictions. Furthermore, in our long experience GxP compliance & scrutiny are less (thus cheaper) in developing countries, than in developed ones. So future American plants can expect higher costs of compliance and more scrutiny.
Of course, some big pharma firms are now pledging to boost output in America; but “there is many a slip between cup & lip”. And the time for a new plant to come on stream would likely be after the end of Mr Trump's second term, during which trade policies could morph again!

Meanwhile, the American pharma giants which have the great majority of their sales in the USA will take a big hit. Ultimately, this will increase prices elsewhere, slow the creation of new medicines, & increase the costs of any imports climbing a American tariff wall.
The Editor
FACING DISASTER: Knowledge and Experience Count

By Bill Torpey
B. Sc in Applied Biology. 40 years experience in pharma & healthcare in various multinational & small companies as production manager working across the dosage forms. Recently he spent 8 years working with QA on validation
There were £2.75 million worth of active ingredients in the freeze dryers when the cyclone struck.
The Emergency call came through to me about 5:00 in the morning from the very stressed shift electrician, to say that all the alarms on the freeze dryer control panels were lighting up. “A bit like a Christmas tree, you need to get here Bill.”
As I was the Sterile Products Shift Leader responsible for the bulk processing, sterilising, filling, freeze drying, capping and inspection it was “my baby” to look after.
But although I only live about 3 miles from the factory, the damage that the cyclone had caused meant I took 3 hours on foot to get to the factory, through the dark deserted streets with flying roof tiles, and blocked with fallen trees and cars.

Once in the control room I could see every alarm on both panels flashing “a bit like Chernobyl without the sirens”. But this meant we had not lost ALL power!
However, the shelf temperature in the freeze driers was rapidly rising to near the eutectic point of the rare naturally occurring polypeptide hormone. Without action this API would boil over under vacuum i.e. evaporate.
So, I accepted all alarms which allowed me to restart the freezing of the shelves. After about 2 hours I then restarted the lyophilisation cycles.
I found out later that the active ingredient had not suffered due to the breakdown or become denatured. Both batches passed QC analysis for potency and other criteria and were released for sale! It was very gratifying to have used my experience & knowledge. I was glad I’d kept up to date with training & techniques and used outside specialists.
Bill Torpey
Yes Cyclones & Storms do happen

Currently there are again deadly floods along the French Riviera with electricity & water outages. 10” of rain fell locally in 1 hour. Bad storms also hit SW France on 20th May disrupting rail connections.
Record-breaking floods in eastern Australia have killed four people and stranded tens of thousands after days of relentless rain across New South Wales, the country’s most populous state. Entire towns remain cut off and roads submerged after a powerful weather system dumped 6 months of rain in three days (20-23rd May).
Do you have an “Emergency Plan”?. I don’t mean the 27page protocol with appendices which will take 15minutes to read (after you have found it) but a simple clear understanding of immediate actions to take concerning your “patch”.
The Editor
Due Diligence versus Audit/Inspection

By Richard van Duyse
Experienced international vaccine industry consultant. Specialized in project & operational management, facility design, start-ups, & technology transfers. Bachelor of Applied Science (B.Sc.).
Life Sciences is long-term type of industry, but we’re in a period of change & disruption. Given current geopolitics many pharma/biological/vaccine companies are reconsidering their strategy concerning the location of their businesses and the manufacture of their products. This can involve new buildings, refurbishment of existing ones, or purchase of a 3rd party business or its facilities.
Once a company has taken decisions on location (after tax, tariffs, market size & access considerations) the selection can start. Existing facilities (whether or not functioning) will often be considered first. But before the company can consider purchasing another life science company or facility it needs a thorough check out. Such a purchase will require a Due Diligence Process and this is far wider ranging than an Audit or Inspection.
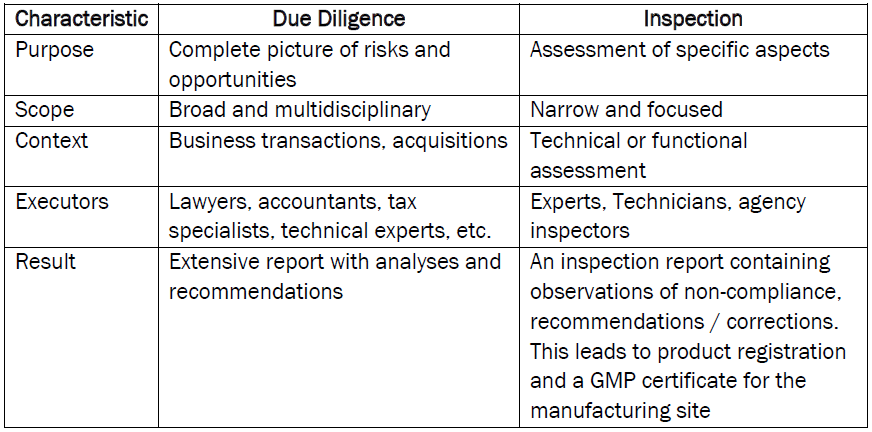
The differences between due diligence and an audit/inspection lies mainly in the scope, purpose and context in which they are carried out.
1. Due Diligence requires Thorough and Multidisciplinary Research
It’s is an extensive and systematic investigation to obtain a complete picture of the company/site, before takeovers, investments or mergers, & includes:
- Financial health
- Legal obligations
- Tax structures
- Operational and quality management processes
- People
- Commercial position
- Buildings, equipment, technical condition of etc.
- Product Registrations and their status of registration with respect to current practice.
This process helps to identify risks, opportunities and hidden problems, so that well-financial decisions are made knowing what additional personnel, facility, quality systems, & commercial costs may be involved.
Due diligence should provide a systematic evaluation of all areas of a third party’s activities, for the purchaser, mapping out the risks and opportunities; to avoid surprises, & plan for a coherent take-over post approval.
2. Audit/Inspection: Targeted and Limited Investigation
An inspection (alternatively called a factory audit) is usually a specific and limited investigation, often focused on one aspect such as the technical condition of a building and equipment, the quality system, the operation of installations or compliance with the applicable regulations such as GMPs. It is a “photo” of examples of a company’s activities in the areas that of interest.

It is not comprehensive but assumes that the condition of the items and activities that are evaluated show the overall condition of the company being inspected/audited. Inspections/audits are carried out by specialists and they are intended to assess the physical or functional condition of a facility, the quality system and/or processes.
In the pharma / vaccine industry, manufacturers of medicines, active substances, & biological products for humans and animals (regularly) undergo a GMP inspection to obtain a manufacturing and/or marketing license or to maintain them in order to prove that they can continuously guarantee quality and regulatory compliance.
These inspections are carried out periodically by Regulatory Agencies. However, as a key part of supplier management, companies perform inspections/audits of manufacturers and service providers when, for instance, they have placed product manufacture with a CMO (contract manufacturing organization) or they have taken products from a third-party company via Technology Transfer. Suppliers of key starting materials, components or services will also be inspected/audited by their clients.
Inspection/audit is a targeted investigation to check a third party’s ability to consistently provide the item; product or service under the expected contracted conditions. Or in other words; an independent investigation of a facility, product or a combination of products, for which an evaluation is performed to ensure compliance specifications, procedures, regulations, standards, contracts, agreements or other criteria. Carrying out inspections is a recurring activity.
3. Summary of Differences
4. Conclusion
Although both processes are aimed at obtaining information, due diligence is a comprehensive, multidisciplinary investigation used for important business decisions, while an inspection is a focused assessment of specific aspects. Both are very important and necessary to make the right decision.
Richard van Duyse & Steve Biddulph
Flying blind? Less tracking and forecasting
The Editor
Many years ago I travelled to North America with a colleague to discuss a controlled release theophylline deal.
We left London on a mild January day but arrived in New York, where one apartment block which had caught fire was coated in frozen water from the fire hoses. My colleague did not check the weather in North America and had no overcoat during our 3 days in upper NY State.

Everybody (or nearly everybody), understands the value of weather forecasting. What is perhaps less appreciated is the value of epidemic forecasting which is one of the main tasks of America's CDC. The Centres for Disease Control act as USA's public health agency, detecting outbreaks both at home and abroad.
This data collecting is essential for the effective deployment of medication & treatment of diseases (& the CDC funds treatment clinics).
In April’s Newsletter we wrote about the immediate effect of DOGE’s headcount reductions on the CDC's current activities. Unfortunately, it is not only those headcount cuts which will affect this important forecasting institution, but also the long-term effects of the Administrations action in slashing the research funding budgets of the National Institutes of Health (NIH), the National Science Foundation (NSF), the Departments of Defence (DoD) and Energy (DoE).
The president hopes to slash the NIH budget by 38%, ie almost $18 billion. Two months ago, part of that department said it would thus be scrapping 20,000 jobs or 25% of its workforce.
More specific damage is the cancellation of some NIH funded research on vaccines, together with the $11 billion worth of special funds from the CDC for pandemic related research (e.g. including that for wide spectrum mRNA vaccines against the SARS-CoV-2 virus family). Especially dumb are the cuts to programmes which aim to detect mutations which might cause animal diseases to jump to humans.

The NIH is the largest biomedical research centre in the USA. But its day-to-day purchasing of common lab supplies protective clothing consumables & reagents already suffers delays. The NIH also gets blowback from the Administrations attack on universities. For example $400m grants to Colombia including for research on Alzheimer's, Schizophrenia and HIV are threatened. With HIV research cut and fewer treatment clinics expect HIV rates to rise in USA, as in Africa where USAID money halted.
(PS. Direct Weather Forecasting & long term research have also been hit by 1300 job cuts (>10%) at the National Oceanic and Atmospheric Administration).
The Editor





