Global Pharma Under Pressure: Risks, Realities & Rapid Change

Galenisys Newsletter : December 2025
Table of Contents
-
TALES FROM FARAWAY PHARMA
By Steve Biddulph -
WHAT PANDEMIC ?
By The Editor -
VALIDATION, WHOSE BABY IS IT!
By Bill Torpey -
WHICH STANDARDS DO YOU HAVE?
By The Editor -
EASY COME – EASY GO
By The Editor
TALES FROM FARAWAY PHARMA

by Steve Biddulph
Fellow of Royal Society of Biology. Board level pharma experience. QSM and Aseptic Manufacturing & Control Expertise. Galenisys Managing Director.
Continuing Tales from Faraway Pharma Steve recounts visiting a country of contrasts, before it was so controversial.
My trip to Venezuela was to visit various companies manufacturing our products under licence.
Amongst these was a company that had its own products but was also a CMO.
Coincidently they were also producing lines for another company that I'd previously worked for.
It’s factory for solid doses was one of the best sites that I'd ever seen. There were clean and well-ordered facilities, shining new equipment, good dust and cross contamination control throughout the facility; together with well-trained people, compliant manufacturing, control, and quality management systems.
The next (separate) company on my list to visit was only two streets away so there was no need to change hotels which is always a positive!
That gave time for a round of drinks before an evening stroll in the tropical forest. During this I noticed a number of large holes in a small cliff face. Ever curious I poked one with a long stick. The largest spider I had ever seen advanced along the stick, which I immediately dropped and retreated with the speed of a Premier League winger.
Another shock arrived the following morning when I was due at the next company. On approaching their site that fronted onto the street, I noticed the facade was severely fissured in many places. In the reception area the walls were partly collapsed and there was dust everywhere.
Apparently there had been a severe ground tremor that had collapsed walls and destroyed one building. In another, the ground floor had given way & part destroyed levels -1 and -2, where personnel were scrabbling in the dust & rubble to recovered materials which would be used for production.
Elsewhere, the site was continuing to manufacture under blatantly unacceptable conditions!

Inevitably our contracts with this company were immediately cancelled.
How strange to find the best and worst examples in Venezuela so close together.
Steve Biddulph
WHAT PANDEMIC?
By The Editor
Preparing (or not) for Disease X
Do you remember COVID-19, & keep the spare masks, or are you “just getting on with the rest of your life” after a “rare black swan event”.
In November the Independent Inquiry (a thriving business sector here in UK), published part 2 of its’ findings into the Governments’ handling of the COVID-19 pandemic, focusing on communication, management, & preparedness.
“Too little too late” was the succinct resume. OK that's just 1 country, but pandemics mean everybody: 200+ countries.
And potential pandemics are bubbling away everywhere. It was fascinating to see footage of the daily meeting at WHO headquarters in Geneva, in which regional specialists give their daily area updates on typically a dozen or two worldwide clusters.
The narrative goes like “12 cases here for (this) virus … 8 deaths there from (that virus)…” and occasionally “... 15 deaths… cause unknown…” as they go through the list.
Disease X is the name given to the future fungus, bacteria, or virus amongst these (?) which succeeds in becoming the next pandemic.
Experts think it will probably be a virus. Those in the Ebola, Xika, Nipah, and Bird Flu families are among the frontrunners.
Plenty of viruses hang out in “reservoirs” that is to say somewhere latent in the vegetation, soil, insects, or animals which don't normally contact many humans. But now more & more cities are pushing into the carriers’ forests, and it’s inhabitants are bumping into the “reservoirs”.
A typical example is Bangladesh where locals collecting syrup from tree wounds leave collecting pots in the forest overnight. “Great” for fruit bats which like the syrup & add their droppings to the pot which can contaminate it with a strain of the Nipah virus (71% fatalities). This strain is more infectious than that which infected pig farmers in Malaysia via their animals.
A “successful” pandemic needs a strain where:
- each human carrier infects >1 other *
- we have no immunity
- it can use airborne transmission
(* Air travel is a great “help” in this respect. But usually there are few airports on forest fringes)

The Nipah outbreaks have been contained, for the moment. These are takeaways from these & other successes:
You need the experts with the facilities and resources to investigate the mechanism of the infection, isolate it for genetic sequencing (staggeringly fast now), plus national capability for track, trace, & lock down implementation. Not forgetting the capability to treat infected patients in isolation.
These “must haves” will only be present if Governments prepare in advance. The “in country” knowledge & lessons need to be shared internationally, with centres like WHO & the US Centres for Disease Control. (Unfortunately, the American President has defunded both, & USAID in part).
Nevertheless CEPI, GAVI, the WHO & other alliances are pushing ahead with synergistic efforts, including the WHO Pandemic Agreement signed in May this year by over 130 countries. (This establishes good principles, however much haggling on the details of the “Pathogen Access & Benefits Sharing system” lie ahead).
What the UK Inquiry has shown is the need for individual Governments to be very prompt & effective in their communication with the public, and high compliance by their populations.
VALIDATION, WHOSE BABY IS IT!
By Bill Torpey
![]()
B.Sc in Applied Biology. 40 years’ experience in pharma & healthcare as production manager working across the dosage forms. Recently he spent 8 years working with Q A on validation
Fred Flintstone, the main character in the original animated series the Flintstones, regularly asked, when moving to the next agenda action, “Whose Baby is It?”.
Validation responsibility on a manufacturing site maintaining GMP compliance can often be perceived, inappropriately, as a series of tests carried out by indirect staff, and as their responsibility.
This is not an appropriate approach. All site functions that are concerned by the process to be validated must take an active role.
There are various ways the Validation Team can change this culture. A hands-on personal approach spending time with the Production and Engineering staff on creating the protocols includes practical points & avoids rewrites and getting stuck.
On many sites it is also sometimes unclear who is responsible for what task/activity during Validation execution.
So, the default way of thinking risks being: “it is the Validation staff”. This can lead to misunderstanding and mistakes. Clear roles and responsibilities for a Validation exercise must be defined to ensure that all activities are covered and managed. The Validation staff obviously have overall responsibility for planning & coordinating the process.
A key way to ensure ALL departments understanding of their part is, I recommend, including their responsibilities in the Validation Master Plan (VMP).
An example of this is the Responsibility Matrix shown roughly stepwise in the table below:
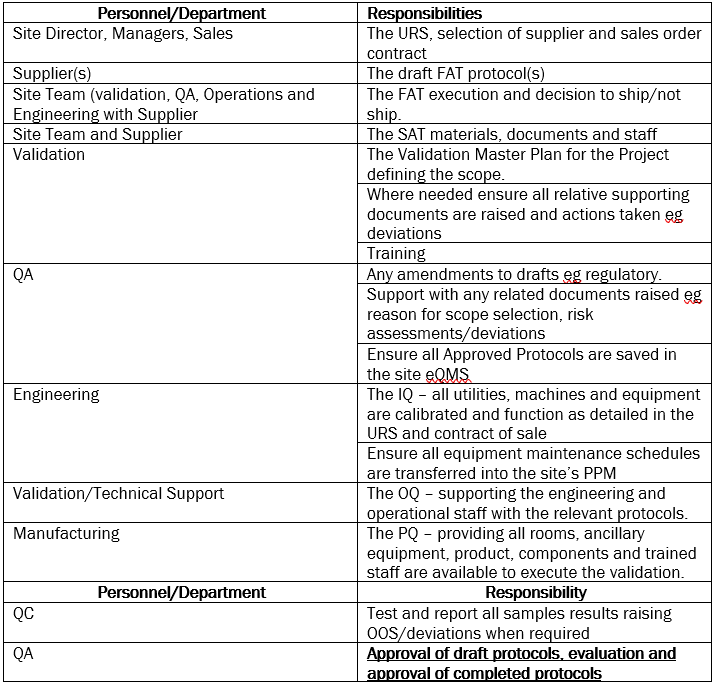
The objective of this approach being that each department completes the section they are responsible for before passing onto the next phase.
Bill Torpey
For more on getting Validation done, enquire about Galenisys Standards
WHICH STANDARDS DO YOU HAVE?
By The Editor
WHICH? is a digital and hard copy consumer magazine here in the UK, and the products they judge “Best Buys” are proud to announce such awards in their sales & marketing.
I was browsing through WHICH? recently and came across their article on multivitamins. The “Best Buy” they recommended in terms of composition and price also happened to include the mention “Manufactured to GMP standard” on the label. Good.
Certainly, this is the first time I have noticed this on OTC nutraceutical and health care products here, although perhaps others also mention it.
How do factories which manufacture regulated & unregulated products decide their stance & practice for Quality Assurance? Is it actively managed when they are part of a Group (perhaps in varying jurisdictions)?
One of our clients some years ago was striving to obtain accreditation on two systems concurrently, one was ISO 9001, and the other (started a bit later) was cGMP. The onsite staff attended training & familiarisation on both (and enjoyed asking trick questions). It took careful coordination as well when creating documentation to suit.
On another site within the same group, the objectives were to manufacture to standards & guidelines emanating from the American Bakers Association for a separate range of their products. (This was before the recent FDA Food Safety Modernization Act “FSMA” was introduced. The ABA as the US Industry body are interacting with FDA on this matter).
This is not the only way to think about this issue. It’s good to consider “SWOP” i.e. System Workload Organisation & People, when auditing. Galenisys found in practise that the deviations noted during these audits are often the result not of the standard which the site has adopted, or is regulated for, but as the result of unsatisfactory personal standards of some members of staff.
The Editor
EASY COME – EASY GO
The ebb and flow of burgers, chicken nuggets, weight loss sales, tariffs & share prices.
The Editor
Paper gains GO. It’s been a bumpy ride for shareholders of Novo Nordisk & Eli Lilly the makers of weight loss drugs. At end July the Danish firm lost 1/4 of its market value in a day after it cut sales and profit forecasts for OZEMPIC. As Eli Lilly has a key rival product, its share price fell by 14%, the biggest one day loss in 25 years.
Ozempic, is one of the appetite suppressing drugs known as GLP-1 agonists and has been prescribed for diabetes since 2017. But then Wegovy (the same API, Semaglutide, as in Ozempic) easily came to the profits rescue becoming approved by FDA 4 years later. Wegovy is aimed specifically at weight loss and in principle will help Health Authorities combat the rising tide of health conditions & welfare costs resulting from obesity. People using this 1st GLP-1 injection lose 15 to 20% of body weight, much more easily than to hard to sustain dieting.
But the disappointing (for the stockmarkets) sales forecasts caused Novo Nordisk, the lighter of the two companies, to also shed its boss recently, because of flagging sales in USA, the world's #1 market and where there’s a lot of overweight people. (His successor was the head of its non-American business).
The trouble was caused by Eli Lilly launching its slightly more effective GLP-1 products Mounjaro and Zepbound in 2022 and 2023. Eli Lilly’s financial results were enviable but overshadowed by clinical trial results showing less slimming power for its new pill version, than hoped for.

EASY GO: Enter the FDA who let others into markets starved of “monopoly” products, as Novo & Eli Lilly had production led shortages early on. The two weight loss heavyweights lost 30% market share in USA to American “Compounders” who in EASY COME mode imported dodgy copycat APIs from CHINA (“Aaargh”) & India. These Compounders (pharmacies) accounted for 30% of all anti-obesity Rxs in America. But “EASY GO” again: in February, the FDA at last took Wegovy off its shortage list.
Meanwhile other big outfits are moving in. For example Pfizer, will acquire Metsera a biotech working on next generation weight loss treatments for $7.3 billion.
Going elsewhere, I was surprised to read that two-thirds of the world’s 1 mio obese adults are in “poorer” countries. This includes India & China where, as we know, burgers, chicken nuggets and other fast foods have found expanding markets & weight effects.
Indian & Chinese pharma companies have seen the opportunities. Ten plus Indian companies have semaglutide versions* in late stage trials. Their products are planned for home & export. (*China is reported to have approx. 20). Eli Lilly, Novo, and Regeneron have all signed licencing deals recently with Chinese companies for promising next generation weight loss candidates.
The ups and downs will no doubt continue since :
- Easier to use weight loss pills are in these major companies pipelines.
Trumpian tariffs come (and go) especially on pharma
Patients who cease their weight loss medication regain some weight if they don’t exercise & police their diets!





